Journal of Animal Health and Production
Research Article
Prevalence of Avian Influenza H5, H7 and H9 Viruses in Commercial Broilers at Karachi, Pakistan
Amjad Ali Channa1, Mansoor Tariq1*, Zaheer Ahmed Nizamani1, Nazeer Hussain Kalhoro2
1Department of Veterinary Pathology, Sindh Agriculture University Tandojam; 2Sindh Institute of Animal Health, Karachi.
Abstract | Avian influenza viruses (AIVs) are highly contagious and pathogenic viruses that caused severe disease in poultry and are potentially zoonotic. Since last decade the outbreaks of AIVs have been increased globally including Pakistan. Therefore this study was conducted to determine the prevalence of avian influenza H5, H7 and H9 viruses in broilers at Karachi, Pakistan. A total of 1920 samples, including each of 960 blood samples and tracheal swabs were collected from broilers and tested through hemagglutination inhibition (HI), AIV-ELISA and RT-PCR. The HI test revealed a significant (P<0.01) difference in the seroprevalence of H9 virus (40.11%), H5 virus (16.88%) and H7 virus (6.25%) respectively. The mean antibody titer against H5, H7 and H9 virus was 4.87, 3.72, and 5.31 log2 respectively. AIV-ELISA revealed that overall prevalence of AIV viruses was 8.44% which was subtyped through RT-PCR that revealed the higher occurrence of H9 virus (5.33%) as compared to H7 (1.16%) and H5 (0.31%). However, the coinfections of H7/H9 (0.73%) was higher than H5/H9 (0.21%) and H5/H7/H9 (0.21%). In conclusion, the H5, H7, and H9 avian influenza viruses are circulating in broilers of Karachi, Pakistan; moreover, the prevalence of H9 virus and the H7/H9 coinfections are common than other subtypes of AIV.
Keywords | Avian influenza, Prevalence, Coinfections, Broilers, Karachi
Received | September 15, 2021; Accepted | October 20, 2021; Published | December 15, 2021
*Correspondence | Mansoor Tariq, Department of Veterinary Pathology, Sindh Agriculture University Tandojam; Email: mtsamo@sau.edu.pk
Citation | Channa AA, Tariq M, Nizamani ZA, Kalhoro NH (2022). Prevalence of avian influenza h5, h7 and h9 viruses in commercial broilers at karachi, pakistan. J. Anim. Health Prod. 10(1): 29-34.
DOI | http://dx.doi.org/10.17582/journal.jahp/2022/10.1.29.34
ISSN | 2308-2801
Copyright © 2022 Tariq et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Poultry is largest subsector of agriculture in Pakistan that has become a balancing force for increasing consumers demand for meat. It contributes 35% of overall meat production in the country with growth rate of 9.1% (GoP, 2019). Despite the rapid growth of commercial poultry in Pakistan, it is influenced by variety of viral and bacterial diseases mainly avian influenza viruses (AIVs) that caused high morbidity and mortality of poultry since last decade. Avian influenza (AI) is a member of family Orthomyxoviridae and was subtyped into 16 hemagglutination (H1-16) and 9 neuraminidases (N1-9). Based on pathogenicity, it has two pathotypes such as highly pathogenic (HP) and low pathogenic (LP), the former have pathogenicity index (P.I) greater than 1.2 whereas latter have P.I lower than 1.2 (OIE, 2015).
Most of the AIV viruses infects poultry are low pathogenic but can be highly pathogenic and zoonotic. Highly pathogenic avian influenza was first time reported in northern areas of Pakistan in the early 90’s (Naeem et al., 1995) afterwards the second outbreak was reported as low pathogenic H9N2 in 1998 that caused 10-20% mortality and decreased egg production up to 75% (Naeem et al., 1999) and then it was reported that the respiratory infections in poultry were associated with H5, H7 and H9 viruses at southern Pakistan during 2007-8 (Ahmed et al., 2009).
Avian influenza (AI) viruses are potentially zoonotic and can cause human infections and was reported that AIV H5N1 virus was isolated from humans mingled at the airport in Thailand during 2005 (Van et al., 2005). Likewise, H7N7 subtype was isolated from 89 infected cases of humans in Netherland (Fouchier et al., 2005; Shahzad et al., 2007). Since, 1997 AIV viruses (H5 and H9) were founded to infect humans in different countries such as Thailand, China, Vietnam, and Indonesia (Mukhtar et al., 2007; Sarwar et al., 2013; Xu et al., 1999). An increase in the outbreaks of AI have been reported throughout the globe and it has now become endemic in poultry in the southern Pakistan and can be zoonotic (Abbas et al., 2010); therefore, it is necessary to screen the prevalence of avian influenza viruses on regular basis. Thus, current study was designed to investigate the prevalence of H5, H7 and H9 viruses in commercial broilers at Karachi, Pakistan.
MATERIALS AND METHODS
Ethical Approval
The current study was approved by Animal Ethics Committee, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University Tandojam, Pakistan.
Selection Of Poultry Farms And Sample Size
A total of 120 broiler farms located at Karachi was selected for this study. A total of 1920 samples, viz., each of 960 blood and tracheal swabs were collected from December 2018 to November 2019. Each 240 blood samples and tracheal swabs were collected in winter (December-February), spring (March-May), summer (June- August) and autumn (September-November) season respectively. The blood samples were used for hemagglutination inhibition (HI) test, while tracheal swabs were adopted for enzyme linked immunosorbent assay (ELISA) and reverse transcription polymerase chain reaction (RT-PCR).
Collection Of Samples
The blood samples were collected from suspected commercial broilers using sterile 3ml syringe from wing vein. The samples were labeled carefully, immediately placed in an ice cooled thermostat and shifted to Research and Development Laboratory of Sindh Institute of Animal Health, Karachi for further analysis.
The blood samples were transferred to sterile Eppendorf tubes, labeled, and centrifuged at 5000 rpm for 5 min. After that the sera were transferred to a new Eppendorf tubes and placed at -20°C for serological investigations.
Hemagglutination Assay
The antibody titers were determined by calculating 4 HA unit (4HAU) as described by Allan and Gough, (1974). Briefly, the HA titer is the reciprocal of the highest dilution of the serum that showed complete inhibition of 4HA antigens such as H5, H7 and H9 respectively.
Hemagglutination Inhibition Test
Avian influenza viruses (AIVs) were subtyped through hemagglutination inhibition (HI) assay. Briefly, a volume of 25 µl of normal saline was dispensed at V bottom shaped Microtiter plate in all wells. After that, placed 25 µl of serum in the first well and make a twofold dilution to 11th well, keeping the 12th well as control. Added 25 µl of 4 HA virus in all wells except the control and incubated the plate for 30 (min) at 4°C. After that added 25 µl of 1% chicken RBC’s across the plate and incubated it for 30 (min) at 4°C. The HI titers are the highest serum dilution causing complete inhibition of 4HAU antigen. The influenza A was subtyped if antibodies were inhibited by biologically characterized viruses such as A/Chicken/Pakistan/NARC-2238/06 (H5N1), A/Chicken/Karachi/SPVC-3/2004(H7N3) and A/Chicken/Pakistan/2/1999(H9N2). The serum was considered as positive having inhibition of antigen a higher dilution of Log2 3 (OIE., 2015).
Enzymed Linked Immunosorbent Assay
The tracheal swabs were tested through ELISA containing polyclonal antibodies coated ELISA plate as per manufacturer’s protocol (Shenzhen China). Briefly, five two-fold dilutions of original standard (20 ng) were prepared. The samples were diluted five-fold by adding 10 µl of each sample and 40µl of sample diluent. Then 50 µl each of standards and samples were dispensed in respective wells. After that the plate was covered with polyethene membrane, incubated for 30 min at 37°C and then washed 3 times by adding 10 µl washing buffer solution to each well. After that 50 µl of HRP-conjugate was dispensed in each well. Then the ELISA plate was covered with membrane and incubated for 30 min at 37°C and then washed three times. Furthermore, 50 µl each of chromogen A and B were dispensed in all wells and the plate was incubated at room temperature in dark room for 10 min. After that 50 µl of stop solution was added, OD450 value was noted and sample to positive standards ratio was calculated by using formula:
S/P value = [1-(OD sample/OD positive control)/ OD negative/OD positive control)] × 100
If S/P values > 0.25 the sample is positive and negative if S/P value was < 0.25.
Extraction Of Rna And Rt-Pcr
The RNA was extracted from AIV-ELISA positive samples through TRIzol® reagent. The RNA was tested through 1-step Hot Start RT-PCR kit (Thermo Scientific US) as per manufacture protocol using primer and proto
Table 1: Primers used in current study.
| Name of virus | Primer Sequence | Product size | Reference |
|
Avian Influenza virus H5-F H5-R |
5'ACAAAGCTCTATCAAAACCCAAC3' 5'TACCCATACCAACCATCTACCAT3' |
499 |
|
|
Avian Influenza virus H7-F H7-R |
5'CAGGCGGAATTGATAAGGAG3' 5'TGCCCCATTGAAACTGAAAG3' |
409 |
|
|
Avian Influenza virus H9-F H9-R |
5’ATCGGCTGTTAATGGAATGTGTT3’ 5’TGGGCGTCTTGAATAGGGTAA3’ |
221 |
Table 2: Seasonal seroprevalence of avian influenza H5, H7 and H9 viruses analyzed by hemagglutination inhibition test in broilers at Karachi.
| Season | No. of farms | No. of samples |
H5 n (%) |
H7 n (%) |
H9 n (%) |
X2 |
P-value |
| Winter | 30 | 240 | 5 (20.84) | 1 (0.42) | 76 (31.67) | 37.23 | 0.0001 |
| Spring | 30 | 240 | 13 (5.42) | 23 (9.59) | 89 (37.09) | ||
| Summer | 30 | 240 | 110 (45.84) | 25 (10.42) | 93 (38.75) | ||
| Autumn | 30 | 240 | 34 (14.17) | 11 (4.59) | 127 (52.92) | ||
| Total | 120 | 960 | 162 (16.88) | 60 (6.25) | 385 (40.11) |
Table 3: Seasonal distribution of mean antibody titers (log2) against avian influenza H5, H7 and H9 viruses in broilers at Karachi.
| Season |
Mean antibody titers (Log2) in broilers |
||
| H5 | H7 | H9 | |
| Winter | 8.00 | 3 | 5.13 |
| Spring | 3.15 | 3.17 | 4.71 |
| Summer | 3.90 | 5.6 | 6.31 |
| Autumn | 4.44 | 3.09 | 5.09 |
|
Average (Log2) |
4.87 | 3.72 |
5.31 |
Table 4: Prevalence percentage of avian influenza H5, H7 and H9 viruses and coinfections occurred in tracheal swab samples of commercial broilers.
| Seasons | No. of Samples | ELISA +ve | Number of RT-PCR positive samples | ||||||
| n (%) | H5 | H7 | H9 | H5/H9 | H7/H9 | H5/H7/H9 | |||
| Winter | 240 | 24 (10) | 0 | 3 | 19 | 0 | 2 | 0 | |
| Summer | 240 | 17 (7.08) | 0 | 2 | 13 | 0 | 2 | 0 | |
| Spring | 240 | 26 (10.83) | 2 | 4 | 17 | 1 | 1 | 1 | |
| Autumn | 240 | 14 (5.83) | 1 | 2 | 7 | 1 | 2 | 1 | |
| Total (%) | 960 | 81 (8.44) | 3(0.31) | 11((1.16) | 56(5.33) | 2(0.21) | 7(0.73) | 2(0.21) | |
| p-value | ns | ** | *** | ns | ** |
ns |
|||
ns = nonsignificant; ** = significant (P < 0.05); *** = highly significant (P < 0.01)
cols as described by (Chaharaein et al., 2009; Ahmed et al., 2009) (Table 1). Briefly, a final volume of 50 μl RT-PCR reaction was prepared containing verso enzyme (1 μl), RT-PCR master mix (25 μl), RT enhancer (2.5 μl), forward primer (1 μl) and reverse primer (1 μl) (Table 1), RNA template (1.5 μl) and RNase-free water 17 μl. RT-PCR amplification was performed as one cycle of cDNA synthesis (50°C for 15 min) and verso inactivation (95°C for 15 min). After that 35 cycles of denaturation (95°C for 20s), annealing for H5, H7 and H9 was done at 59°C, 60°C and 58°C for 30s respectively. The extension was performed at 72°C for 1 min and the final extension was done at 72°C for 7 min.
Statistical Analysis
The collected data was tabulated on Microsoft Excel Sheet; the prevalence (%) and mean antibody titer was calculated. The significant difference (P<0.05) was determined by Chi square by column statistics through Graph Pad Prism-5.0 software.
RESULTS
Results revealed that the overall seasonal seroprevalence was significantly higher (P<0.01) for H9 virus (40.11%) as compared to H5 virus (16.88%) and H7 virus (6.25%) in broilers (Table 2).
The results of mean antibody titer in broilers against avian influenza H5, H7 and H9 viruses were 4.87, 3.72 and 5.31 log2 respectively. Furthermore, the titers against H5 were higher in winter (8 log2) whereas against H7 and H9 were higher in summer (5.6 and 6.31 log2) (Table 3).
The results of AIV-ELISA revealed that the 8.44% of tracheal swab samples were found positive with AI viruses. Furthermore, subtyping through RT-PCR showed that the H9 virus (5.33%) was significantly highest (p≤0.05) followed by H7 (1.16%) and H5 (0.31). Although the coinfection of H7/H9 (0.73%) was comparatively higher (p≤0.05) as compared to H5/H9 (0.21%) and H5/H7/H9 (0.21%) respectively, (Table 4). The avian influenza H5, H7 and H9 viruses were amplified by PCR and observed the PCR product with amplicon sizes such as 499, 409 and 221bp respectively (Figure 1).
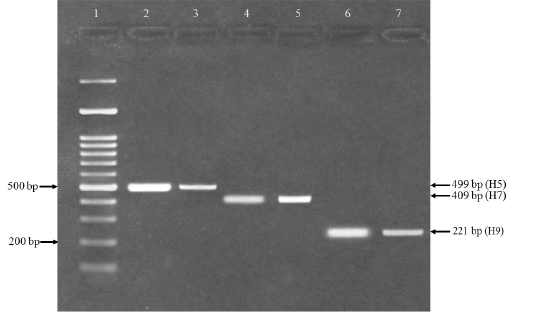
Figure 1: RT-PCR for confirmation of avian influenza H5, H7 and H9 viruses. Lane 1 = 100bp DNA marker (Fermentas, USA), lane 2 and 3= An expected PCR product size 499 bp was detected positive for influenza (H5), lane 4 and 5 = An expected PCR product size 409 bp positive for influenza (H7) and lane 6 and 7 = An expected PCR product size 221 bp positive for influenza (H9).
DISCUSSION
The outbreaks of AIVs have been increased in poultry in southern Pakistan and it has caused high mortality in the poultry. Although the severity of the disease was varied with the species of the birds, like wild water geese infected with AIVs might remain asymptomatic and act as natural hosts (Bergervoet et al., 2017). The respiratory infections have caused drastic damage to the poultry industry in Pakistan since 90’s and was confirmed as low pathogenic H9N2 virus (Naeem et al., 1995). It was then reported by different researchers (Ayaz et al., 2017). Low pathogenic AI caused less mortality, but reduced the poultry production (Soomro et al., 2016). The AIV outbreaks have been increased, it has become endemic in poultry at southern Pakistan and zoonotically potential therefore, it is necessary to know the prevalence of AI viruses in commercial broilers in Karachi, Pakistan (Abbas et al., 2010).
The results showed that the seroprevalence of AIV-H9 virus was most common and, in some cases, AI H5 and H7 viruses as well. Interestingly, the seroprevalence of H5 and H7 viruses were recorded higher in the summer whereas H9 virus was higher in autumn. It has been found the seroprevalence of H9N2 virus in domesticated birds in Pakistan was 53% (Kausar et al., 2018). Correspondingly, the overall seroprevalence of AIVs was 65.2% of which higher prevalence of H9 virus (62.0%) and H5 virus (6.9%) (Chaudhry et al., 2021). Contrary to the current study, Hassan et al. (2020) have found the higher prevalence of H5 virus (71.4%) in chicken and 61.7% in ducks as compared to the H9 virus (28.5%) chicken and 36.8% in ducks in Bangladesh. Similarly, the seroprevalence of H9N2 virus (60%) in the commercial layers at northern Pakistan (Hira et al., 2017). Correspondingly, it has been reported that the seroprevalence of AIV viruses was (14%) in broilers at Quetta Pakistan (Arif et al., 2015). Although, the higher seroprevalence of AIVs was reported in spring and summer (35 and 32%) whereas in winter and autumn was (23 and 10%). It would be interesting to know that whether higher prevalence of AIV H9 viruses might be due to earlier infections or due to other factors such as environmental conditions, sample size and region selected.
Interestingly, the mean antibody titers was found higher against H9 virus as compared to H5 and H7 viruses in broilers. Correspondingly, it has been reported that the mean titers against H9N2 virus in commercial layers was 5.37 log2 (Hira et al., 2017). Likewise, the antibody titers against H9N2 and H7N3 (7.81 and 5.06 log2) was reported in naturally infected poultry Muneer et al. (2001).
The results of AIV-ELISA revealed that the overall prevalence of AI viruses were 8.44%. Moreover, the subtyping through RT-PCR showed that the higher prevalence of H9 virus as compared to H7 and H5 viruses. Although the coinfections of H7/H9 were comparatively higher followed by H5/H9 and H5/H7/H9. Similarly, it has been reported that the prevalence hemagglutinating viruses was 0.02%, whereas H9N2 in broilers were 3% (9/300) Sarwar et al. (2013). Correspondingly, the co-infections of AIVs were 3.2% in poultry out of which the 86.7% of coinfections were classified as H5+H7 (6.7%) and H7+H9 and 13.3% (Karlsson et al., 2019). However, the coinfections in broilers include H5N1/H9N2/ND/IB, H5N1/ND, H5N1/H9N2/ND, H9N2/IB, and H9N2/ILT with proportion of 2.6% each whereas commercial layers include H9N2/IB and H5N1/H5N8/H9N2 was recorded 9.1% in each farm (Shehata et al., 2019). The AI H9 virus was commonly circulating that remains unrecognized and it would be interesting to know whether AI viruses caused respiratory infections or not, though the vaccine for H9 virus is used. However, the coinfections might be due to the simultaneous infection of two or more AI viruses or it can be due to the super infections of circulating AI viruses.
CONCLUSION
Avian influenza H5, H7 and H9 viruses are circulating in broilers of Karachi, Pakistan. A comparatively higher prevalence was recorded for H9 virus and the H7/H9 coinfections as compared to other subtypes of avian influenza.
acknowledgements
The authors thank their respected Sindh Agriculture University Tandojam and Sindh Institute of Animal Health Karachi.
conflict of interest
The authors declare that there is no any conflict of interest.
authors contribution
All authors except first one had supervision contribution. The first to fourth author had the major contribution in paper writing, editing, and reviewing, in addition the role of the fourth author in practical part implementation and finally corresponding by the second author.
REFERENCES





