Journal of Infection and Molecular Biology
Short Communication
Seroprevalence of Newcastle Disease Virus (NDV) in Commercial and Domesticated Birds: during Current Surge of NDV in Pakistan
Aziz-ul-Rahman1*, Momena Habib1, Tayyaba Riaz1, Behzad Hussain1, Farooq Yousaf2, Muhammad Saqalein3, Muhammad Hidayat Rasool3
1Department of Microbiology, University of Veterinary and Animal Sciences (UVAS), Outfall Road Lahore 54600, Pakistan; 2Veterinary Research Institute (VRI), Ghazi Road Lahore 54000, Pakistan; 3Department of Microbiology, Government College University (GCU), Allama Iqbal Road, Faisalabad 38000, Pakistan.
Abstract | A cross sectional study was conducted to evaluate current immune status of various commercial and domesticated birds, including broiler, layers, backyard poultry and other domesticated birds (Pigeon, Peacock, Turkey). Of the total serum samples (n = 452) obtained from 113 flocks, only 372 samples were seropositive to NDV using competitive ELISA. An overall prevalence of 82.3% was noted in studied birds across the country. Seropositivity was more frequent in Khyber Pakhtunkhwa (84.5%) followed by Baluchistan (83.3%), Punjab (82.3%) and Sindh (79.4%). Among the bird types, seropositivity was higher in domesticated wild birds followed by rural birds, commercial broiler and layers (88.6%, 83.9%, 81.9% and 73.3%), respectively. An incresaed seroprevalence provides a clear evidence of exposure to NDV with history of previous outbreak in these farms. Except from commercial broiler and layers, ND vaccine was not given to the birds and high titer coupled with disease occurrence notifies the vaccine failure in commercial birds. In the absence of the vaccine, the results provide a clear evidence of exposure to NDV to wild domesticated birds. Further studies are required to determine the strains circulating for appropriate preventive and control measures.
Keywords | Newcastle disease virus, Khyber Pakhtunkhwa, Punjab, Baluchistan, Sindh, Seropositivity, cELISA
Editor | Tahir Yaqub, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | November 05, 2016; Accepted | December 15, 2016; Published | December 17, 2016
*Correspondence | Aziz-ul-Rahman, Department of Microbiology, University of Veterinary and Animal Sciences (UVAS), Outfall Road Lahore 54600, Pakistan; Email: drazizangel@gmail.com
Citation | Aziz-ul-Rahman, Habib M, Riaz T, Hussain B, Yousaf F, Saqalein M, Rasool MH (2017). Seroprevalence of newcastle disease virus (NDV) in commercial and domesticated birds: Pakistan during current surge of NDV. J. Inf. Mol. Biol. 4(4): 54-59.
DOI | | http://dx.doi.org/10.14737/journal.jimb/2016/4.4.54.59
ISSN (Online) | 2307-5465; ISSN (Print) | 2307-5716
Copyright © 2017 Aziz-ul-Rahman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Pakistan has an agriculture based economy with livestock and poultry as an integral part of it. Nearly every family in the rural areas and every 5th family in the urban areas is associated with poultry in one way or the other (Sadiq, 2004). In Africa and Asia, Newcastle disease (ND) is a major constraint against the development of both industrial and village poultry production (Alders et al., 2001). With all this, the poultry has emerged as second largest industry in Pakistan compensating the protein needs of the country in the form of eggs and meat; annual increase is 4% (Numan et al., 2005). Among the prevailing infectious disease, ND is the most important cause of mortality in chickens (Nguyen, 1992) and many species of domesticated and wild birds have been found susceptible to this disease (Pearson and McCann, 1975; Arshad et al., 1988; Wernery et al., 1992). The spread of ND in areas is normally via newly introduced birds, selling or giving away sick and carrier birds (Tu et al., 1998).
All species of birds, including chicken, pigeon, turkey and wild captive birds are susceptible to NDV (Wambura, 2010), and infection has been reported being transmitted from one type of bird to another without prior adaptation (Abu Elzein et al., 1999; Roy et al., 2000). Some work has been reported about the nature of NDV strains and its biology from the outbreaks occurred in commercial poultry and clinically healthy backyard poultry (Munir et al., 2012).
Appropriate vaccination and subsequent effective immune response are known to be the only safeguard measure to avoid outbreaks; however, evaluation of protective immune response is not well in practice in Pakistan, most of domesticated birds remained ignored even for vaccination. Lack of or ineffective immune response along with frequent NDV vaccination of the birds, particularly in domesticated birds, could be the reason for escape mutants and subsequent novel genotypes (Munir et al., 2012). Serology is of value in determining the status of ND where rural and domesticated wild birds are not vaccinated in routine added with limited virus isolation and subsequent characterization facilities throughout Pakistan. A little or nothing has been reported on distribution of antibodies to NDV in captive wild birds, pigeons, as well as commercial and backyard poultry. In view of this situation, the primary intention of this study was to determine the prevalence of antibodies to NDV at a different locale of commercial (with a history of previous outbreak) and domesticated bird, in Pakistan to get an idea about the current status of disease.
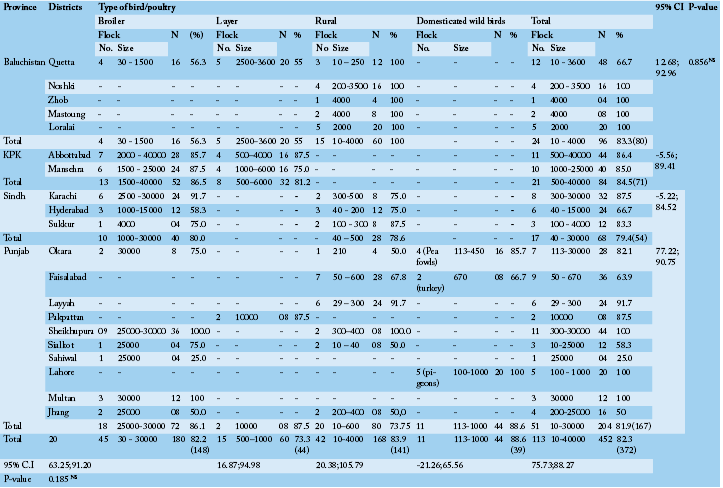
A cross sectional serological survey was conducted, between May to August 2012, to determine the prevalence and distribution of antibodies to NDV, where outbreaks has been observed in different areas. A total of 113 flock from various districts (n = 20) of four provinces of Pakistan, including Punjab, Sindh, Khyber Pakhtoon Khawa and Balochistan was examined, respectively (Table 1). A total of 452 blood (3-5mL) samples from flocks commercial (n = 60), rural (n = 42) and domesticated birds (pigeon, turkeys and peacocks, n = 11) were collected aseptically from the brachial vein of each bird (n = 4 from each diseased flock) and wed to clot at room temperature. The serum was separated, collected in a labeled microfuge tube (1.5mL) and store at -20 0C till further analysis. The survey comprised a detailed questionnaire to evaluate the previous history regards the age of infection, clinical symptoms, mortalities and course of infection and management practices on farms.
All the serum samples were analyzed for NDV antibodies using commercially available competitive ELISA kit (ID screen(R) Newcastle Disease Competition, France). Briefly, 20uL of each serum sample (20uL and 80uL of dilution buffer) and each of negative and positive controls (100uL) were added to corresponding microplate well containing coated antigen, and incubated for 30min at room temperature. Post washing thrice, 100uL of anti-NDV conjugate was added to each well and incubated for 30 min at room temperature followed by washing three times and addition of 100uL of substrate to each well for 10 min. The enzymatic reaction was stopped using 50uL of stop solution and optical density of each well was recorded using ELISA reader (Multiskan EX, Thermo Electron, Finaland). The procedure performed and the results obtained were validated as per kit provided validation readings. The percent inhibition (PI) was calculated as follows:

Serum samples with PI greater than 40 were considered positive, doubtful if is between 30 to 40 and negative if PI is less than 30. Each serum sample was tested twice and there was a complete concordance in results for each time of serum analysis.
The seroprevalence of NDV infection on basis of bird’s types and locale was compared by means of the Chi-square test. A significant level of 5% was used.
Antibody evidence of Newcastle disease virus infection was found in 372 sera of the 452 total birds comprising of commercial layer (n = 60), broiler (n = 180) and rural (n = 168) and domesticated wild birds (n = 44). The domesticated wild birds included pigeons (n = 20), turkeys (n = 8) and peacocks (n = 16) throughout all 20 geographic regions of the survey. The birds were of varying ages e.g., broiler from day 18 to 38, layers from a week 7 to 41 weeks, rural birds from a week 4 – 36, and domesticated turkeys, pigeons and peacocks were from a week 21 – 56, 24 – 37, and 48 – 76, respectively. Among the flock examined, only commercial layers and broilers were with history of NDV oral vaccine. Neither of the rural and domesticated wild birds was vaccinated or at least the farmer/owner was ignorant of the status. The birds have been observed with pin point hemorrhages in proventriculs, hemorrhages at ceacal tonsils, greenish diarrhea, and respiratory distress, torticollis, head tossing, watery nasal discharge, and muscular tremors as described by Alexander (2001) with flock mortality in the range of 30 – 60% for broilers, 25 – 40 % for layers, 40 – 70% for rural birds and 60 – 80% for domesticated wild birds in a previous outbreak. The complete flocks and density is indicated in Table 1.
The distribution of NDV antibodies was high in all the provinces (82.3%; Overall); geographically it was highest in KPK (84.5%) followed by Baluchistan (83.3%), Punjab (81.9%) and Sindh (79.45%). These results are in agreement with previous studies those detected antibodies against NDV on the basis of geographical occurrence, similar as in Bangladesh (88%; Biswas et al., 2009) and in Ecuador (97%; Sonia et al., 2006) but much higher as compared to what has been reported 46.1% in Tanza
nia (Yongolo et al., 2001), 36.9% inZambia (Alders et al., 1994) and 27% in Zimbabwe (Kelly et al., 1994). In spite of vigorous vaccination schedules, ND is stilled a havoc in the poultry industry of Pakistan and a number of outbreaks have been recorded even in vaccinated chicken flocks (Siddique et al.,
N, Total number of birds; NS, Non-significance (P> 0.05); CI, Confidence Interval
1986). In an unvaccinated flock, positive serology and clinical signs are strong diagnostic evidence of ND infection (Alexander, 2001).
Among different bird categories, seropositivity was more frequent to domesticated wild birds (88.6%) followed by rural birds (83.9%), commercial broilers (82.2%) and layers (73.3%), respectively (Table 1). For antibody titer to NDV, a relatively high percentage of bird showed increased percent inhibition from 81 to 100. With this much concentration of antibodies, the percent inhibition to NDV sample antibodies in domesticated birds was higher than broiler (52.8%), layers (50.0%) and rural birds (42.9%) (Table 2).
As concern with the commercial birds, 82.2% seroprevalence has been observed in broiler comprising 86.5% in KPK, 86.1% in Punjab, 80% in Sindh and 56.3% in Baluchistan Provinces of Pakistan. The findings of the present study are in agreement with the results of a previous study, in which ranging from 5 to 83% seropositivity has been recorded in broiler population from different regions of Morocco (Bell and Moulodi, 1988). Mozaffor et al. (2010) found that 78.04% samples of broilers were positive for NDV antibodies in Bangladesh which is slightly lower from the present findings. Similar reports have been described by Numan et al. (2005) who reported that 98.07% of serum samples were positive for antibodies to NDV for broilers in Punjab Province, which is actually higher than as present study finds (86.5%) in the same region.
As compared to broiler low seroprevalence (73.3%) was observed in layer chickens with highest in Punjab (87.5%) followed by 81.2 % in KPK and 55% in Baluchistan. In a similar study Yongolo (1996) reported a variable sero prevalence of 25-81.5% in Tanzania and he noted the variation in sero prevalence in different localities. In another study, as compared to our study, higher prevalence of antibodies to NDV (96.67%) has been reported in Bangladesh (Hossain et al., 2010). As similar, Mozaffor et al. (2010) found up to 90% samples of layers were positive for NDV antibodies. As geographical variation in different states within country, Anzaku et al. (2014) reported 37% to 79% seroposotivity of chicken samples against NDV antibodies from various areas. Our findings are in agreement with the result of a previous study, in which 75% antibody base prevalence against NDV has been detected (Tariq and Taib, 2010). In another study, higher seroprevalence (100%) as compared to present findings (73.3%) has been reported in layer chickens against NDV in Pakistan (Numan et al., 2005). But in some previous studies, low seroprevalence 43.68% and 14% has also been observed in the central highlands and Ethiopia, respectively (Japiot et al., 1990; Ashenafi, 2000).
In the present study, the high antibody base prevalence against NDV was observed in wild birds (88.6%) as compared to rural chicken (83.9%). The birds other than domestic chickens have been known to be sources of the spread of ND virus (Roy et al., 1998) in several countries (Alexander et al., 1984). Moreover, the study conducted by Otim et al. (2007) indicated that other domestic and wild birds are among the risk factors associated with ND outbreaks in free-ranging village chickens in Uganda. In another study, conducted by Mai et al. (2004) reported 6.7% of seropositivity against NDV in ducks of Plateau State. Similarly, out of the 205 serum samples collected from guinea fowls, 13.6% had antibodies against the ND virus. Nwanta et al. (2006) concluded that both local ducks and guinea fowls had past exposure of NDV due to the existence of antibodies against the said virus. The significant sero positive rate of NDV in wild birds in the present investigation is indicative of the continuing infection pressure.
The present study showed a relatively higher (83.9%) seroprevalence rate of ND virus antibodies in rural chickens, compared to what has been reported (41%) earlier by Adu et al. (1986), but are in agreement with results of previous studies, 60%, 60.3%, 72%, and 74.3% seroprevalence were reported (Ezeokol et al., 1984; Orajaka et al., 1999; Nwanta et al., 2008; Eze and Ike, 2015) in rural flocks of Nigeria. As similar, Nwanta et al. (2006) reported a prevalence of 73.3% in rural chickens. Our findings are in contrast with previous results that reported 46% positive rate using ELISA (Aziz and Ahmed, 2010) and 37.56% against NDV antibodies in backyard flocks (Hadipour, 2009).
Table 2: Distribution of antibodies in each type of bird according to percent inhibition
|
Percent Inhibition Range |
Broiler N(%) |
Layer N(%) |
Rural N(%) |
Domesticated Wild Birds N(%) |
Total N(%) |
P-value |
|
0-40 |
32 (17.8) |
16 (26.7) |
27 (16.1) |
5 (11.4) |
80 (17.7) |
0.000* |
|
41-60 |
21 (11.7) |
7 (11.7) |
25 (14.9) |
5 (11.4) |
48 (10.6) |
|
|
61-80 |
32 (17.8) |
7 (11.7) |
44 (26.2) |
4 (9.1) |
87 (19.2) |
|
|
81-100 |
95 (52.8) |
30 (50.0) |
72 (42.9) |
30 (68.2) |
227 (50.2) |
|
|
Total |
180 |
60 |
168 |
44 |
452 |
N, Total number of birds; *,Significance (P<0.05)
Based on the findings of the present study, it is concluded that despite of vaccination outbreaks has been observed in different regions which notify the mutation in the field circulating virus. This is a clear indication of high pressure of said virus among birds with possibility of vaccine failure. The vaccine failure due to many reasons, ineffective bird’s immune response, and inappropriate vaccine strains added with frequent vaccination without titer evaluation, particularly in commercial birds provide an opportunity of high rate of genetic change as evidenced and reported by Munir et al. (2012). We also acknowledge that the apparent prevalence of infected flocks in this study is only an estimate and is subject to sampling variation. However, it provides a first estimate of flock seroprevalence that can be used for the planning of future studies. Further studies are required to determine the strains circulating for appropriate preventive and control measures. Management practices such as disease monitoring programs, appropriate prevention, and control measures should be put in place in order to prevent loss of poultry and income due to outbreaks of the disease.
Conflict of interest
The authors have declared that there is no conflict of interests with regard to publication of this paper.
Author’s Contribution
AR apprehended the idea and drafted the skeleton of the manuscript. FY, AR and MS collected the samples from different regions. MH, TR and BH tested samples in the laboratory. AR and MHR did the editing after final checking of errors and all authors approved the final manuscript.
References